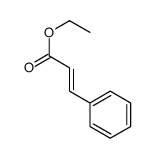
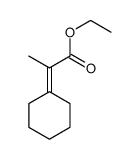
|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
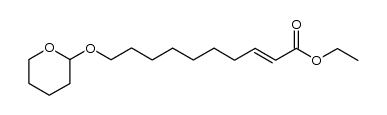
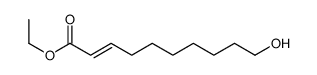
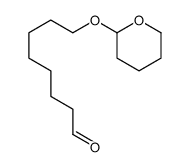
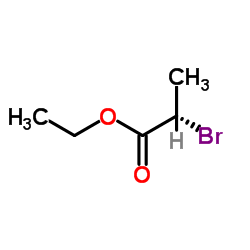
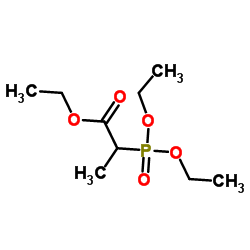
![S-[1-(ethoxycarbonyl)ethyl] diethyl phosphorothioate Structure](https://image.chemsrc.com/caspic/285/22455-04-5.png)
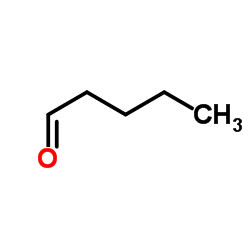
![Ethyl [(ethoxycarbonyl)sulphanyl]acetate Structure](https://image.chemsrc.com/caspic/404/52790-15-5.png)