|
~97% |
|
~95% |
|
~% |
|
~% |
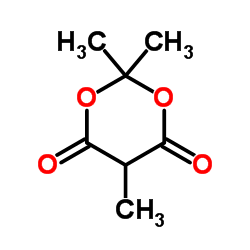
![(3R,4R)-4-acetoxy-3-[(1R)-1-(tert-butyldimethylsilyloxy)ethyl]-2-oxoazetidine Structure](https://image.chemsrc.com/caspic/163/76899-07-5.png)
![5-[(2S,3S)-3-[(1R)-1-[tert-butyl(dimethyl)silyl]oxyethyl]-4-oxoazetidin-2-yl]-2,2,5-trimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/254/156630-83-0.png)
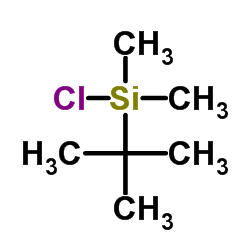
![5-[(2S,3S)-1-[tert-butyl(dimethyl)silyl]-3-[(1R)-1-[tert-butyl(dimethyl)silyl]oxyethyl]-4-oxoazetidin-2-yl]-2,2,5-trimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/216/156630-84-1.png)