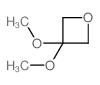
|
~69% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
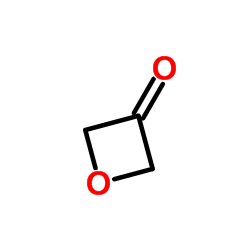
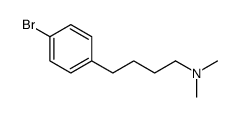
![3-[4-[4-(dimethylamino)butyl]phenyl]oxetan-3-ol Structure](https://image.chemsrc.com/caspic/275/922500-88-7.png)
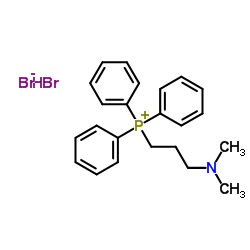
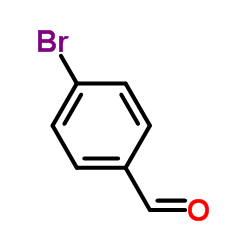
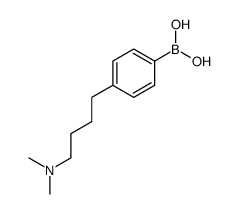
![ethyl 2-[3-[4-[4-(dimethylamino)butyl]phenyl]oxetan-3-yl]acetate Structure](https://image.chemsrc.com/caspic/247/922501-04-0.png)