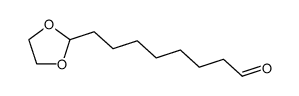
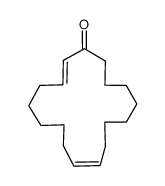
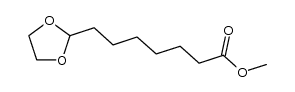
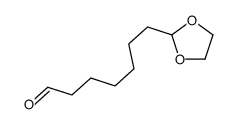
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~72% |
![8-[1,3]dioxolan-2-yl-octanoic acid methyl ester Structure](https://image.chemsrc.com/caspic/047/953-29-7.png)