|
~94% |
|
~72% |
|
~% |
|
~% |
|
~94% |
|
~73% |
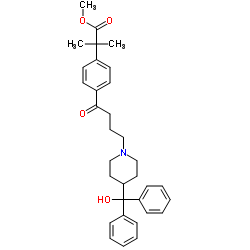
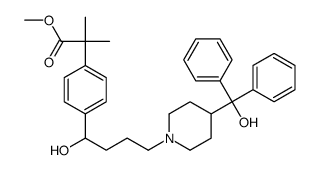
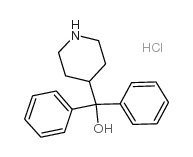
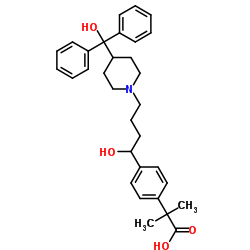
![4-[4-[4-(Hydroxydiphenylmethyl)-1-piperidinyl]-1-butyn-1-yl]-α,α-dimethyl-benzeneacetic Acid Methyl Ester Structure](https://image.chemsrc.com/caspic/048/154825-95-3.png)

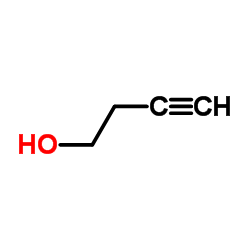
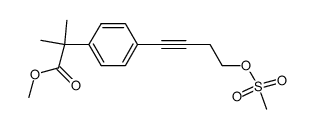
![methyl 2-[4-(4-hydroxybut-1-ynyl)phenyl]-2-methylpropanoate Structure](https://image.chemsrc.com/caspic/124/154825-93-1.png)