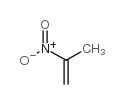
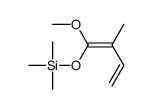
|
~82% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~53% |
|
~% |
|
~% |
|
~% |
|
~% |

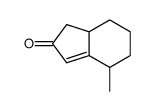
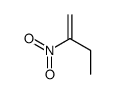

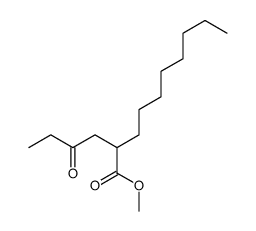

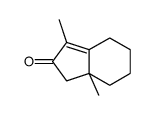
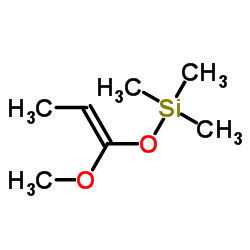
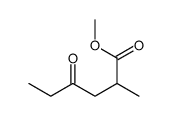
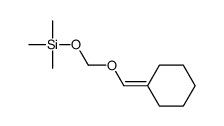
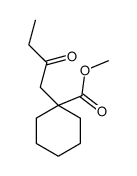
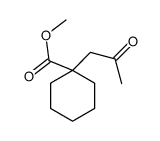
![spiro[4.5]decane-2,4-dione Structure](https://image.chemsrc.com/caspic/347/88869-10-7.png)