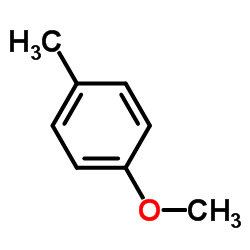
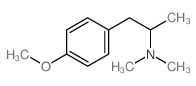
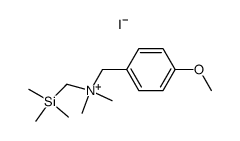
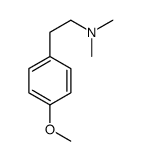
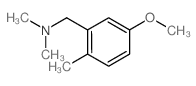
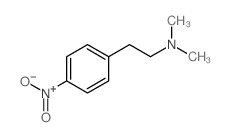
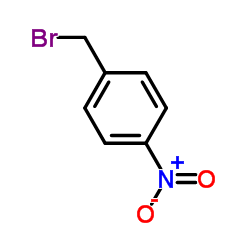
|
~% |
|
~54% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
|
~% |

![4-[2-(dimethylamino)ethyl]benzonitrile Structure](https://image.chemsrc.com/caspic/274/83937-66-0.png)