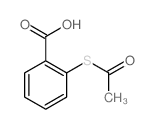
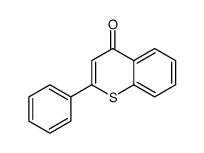
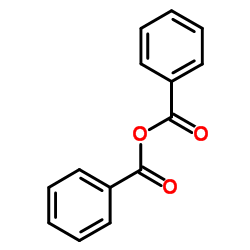
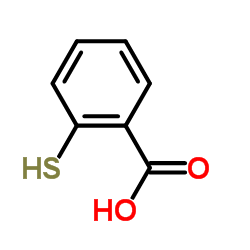
|
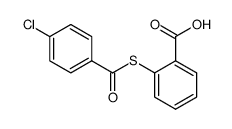
~91% |
|
~% |
|
~% |
|
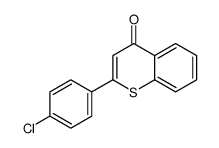
~86% |
|
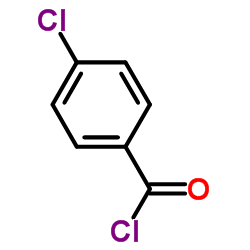
~89% |
|
~% |
|
~% |
|
~% |
![triphenyl[(phenylimino)ethenylidene]phosphorane Structure](https://image.chemsrc.com/caspic/326/64448-06-2.png)