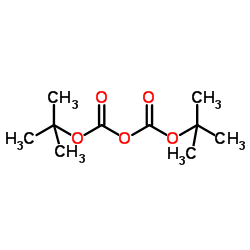
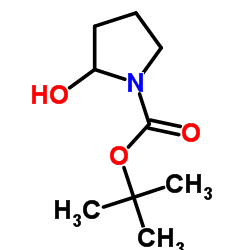
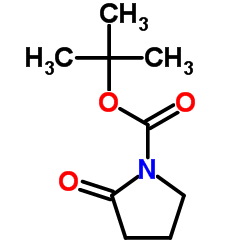
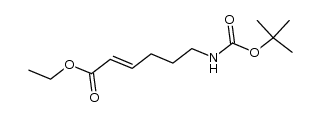
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
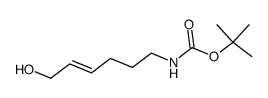
![Carbamic acid, [(4E)-6-oxo-4-hexenyl]-, 1,1-dimethylethyl ester (9CI) Structure](https://image.chemsrc.com/caspic/294/646062-88-6.png)