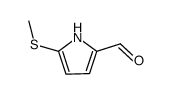
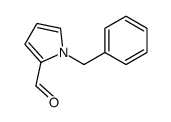
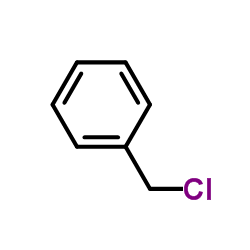
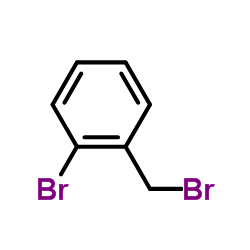
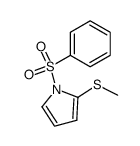
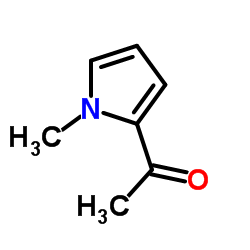
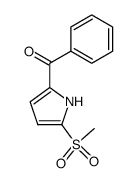
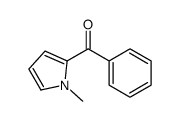
|
~% |
|
~% |
|
~% |
|
~% |
|
~58% |
|
~75% |
|
~99% |
|
~% |
|
~65% |
|
~% |