|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
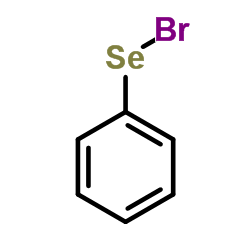
![5-methyl-9-[(2-methylpropan-2-yl)oxycarbonyl]-9-azabicyclo[4.2.1]nonane-5-carboxylic acid Structure](https://image.chemsrc.com/caspic/235/125736-06-3.png)
![9-O-tert-butyl 5-O-methyl 5-phenylselanyl-9-azabicyclo[4.2.1]nonane-5,9-dicarboxylate Structure](https://image.chemsrc.com/caspic/159/125736-08-5.png)
![9-Azabicyclo[4.2.1]non-2-ene-9-carboxylicacid, 2-acetyl-, 1,1-dimethylethyl ester, (1R)- (9ci) Structure](https://image.chemsrc.com/caspic/293/90741-53-0.png)
![tert-butyl (6R)-5-(1,2-oxazolidine-2-carbonyl)-9-azabicyclo[4.2.1]non-4-ene-9-carboxylate Structure](https://image.chemsrc.com/caspic/452/125736-18-7.png)
![(1R,6R)-2-(2-hydroxyacetyl)-9-Azabicyclo[4.2.1]non-2-ene-9-carboxylicacid, 1,1-dimethylethyl ester Structure](https://image.chemsrc.com/caspic/142/125736-13-2.png)
![9-Azabicyclo[4.2.1]non-2-ene-9-carboxylicacid, 2-[2-[[(1,1-dimethylethyl)dimethylsilyl]oxy]acetyl]-, 1,1-dimethylethylester, (1R,6R) Structure](https://image.chemsrc.com/caspic/180/125736-12-1.png)
![(6R)-9-[(2-methylpropan-2-yl)oxycarbonyl]-9-azabicyclo[4.2.1]non-4-ene-5-carboxylic acid Structure](https://image.chemsrc.com/caspic/104/125736-14-3.png)
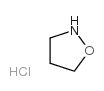
![tert-butyl (6R)-5-carbonochloridoyl-9-azabicyclo[4.2.1]non-4-ene-9-carboxylate Structure](https://image.chemsrc.com/caspic/066/125736-15-4.png)
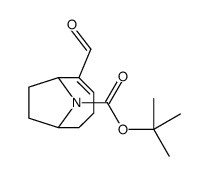
![9-Azabicyclo[4.2.1]non-2-ene-2-carboxaldehyde, (1R)- (9CI) Structure](https://image.chemsrc.com/caspic/011/125736-21-2.png)