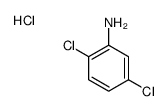
|
~92% |
|
~% |
|
~0% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~% |
|
~94% |
|
~52% |
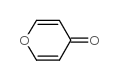
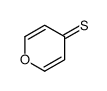
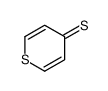
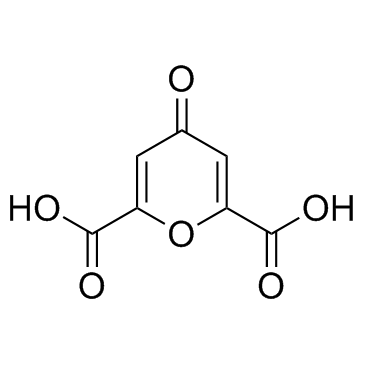
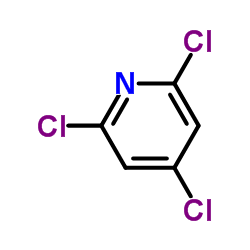
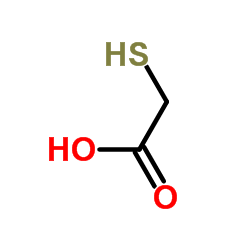
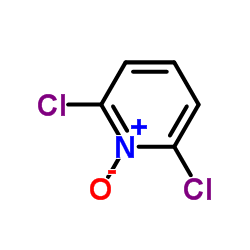
![2-[(2,6-DICHLOROPYRIDIN-4-YL)THIO]ACETIC ACID Structure](https://image.chemsrc.com/caspic/195/80542-50-3.png)
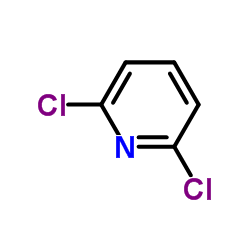
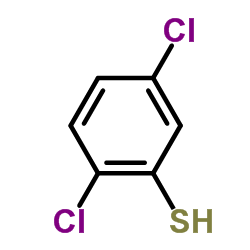
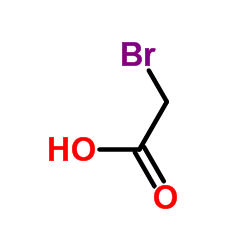
![Acetic acid,2-[(2,5-dichlorophenyl)thio] Structure](https://image.chemsrc.com/caspic/191/6274-27-7.png)