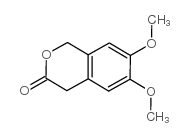
|
~% |
|
~% |
|
~%
Detail
|
|
~% |
|
~% |
|
~% |
|
~27% |
|
~20% |
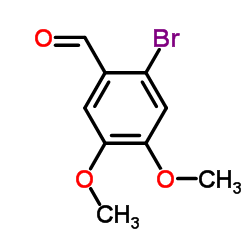
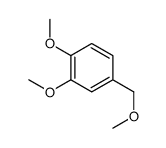
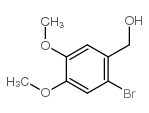
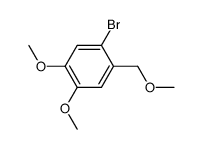
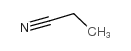

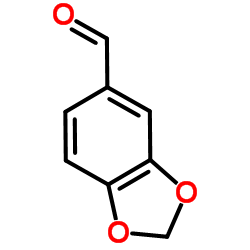
![6-bromo-5-(methoxymethyl)benzo[1,3]dioxole Structure](https://image.chemsrc.com/caspic/299/34679-09-9.png)
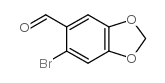
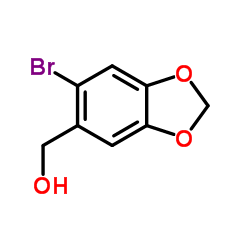

![2-(6-(methoxymethyl)benzo[d][1,3]dioxol-5-yl)acetonitrile Structure](https://image.chemsrc.com/caspic/171/124619-18-7.png)
![7H-1,3-Dioxolo[4,5-g][2]benzopyran-7-one, 5,8-dihydro Structure](https://image.chemsrc.com/caspic/238/34140-20-0.png)