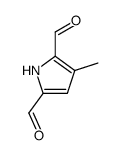
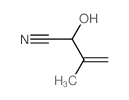
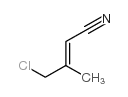
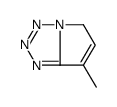
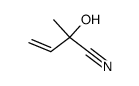
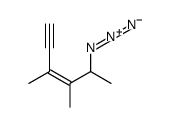
|
~35% |
|
~25% |
|
~57% |
|
~% |
|
~% |
![6H-Pyrrolo[1,2-c][1,2,3]triazole,4-methyl-(9CI) Structure](https://image.chemsrc.com/caspic/196/64804-02-0.png)