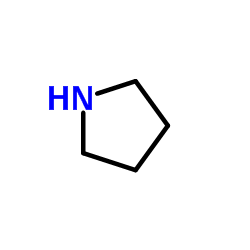
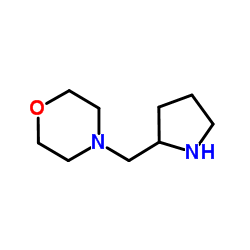
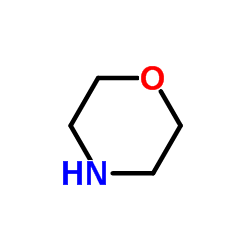
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![2-(R,S)-5-(S)-[3.3.0]-1-aza-2-thia-3-oxabicyclooctane-2-oxide Structure](https://image.chemsrc.com/caspic/117/1013941-83-7.png)
![1-[[(2S)-pyrrolidin-2-yl]methyl]piperidine Structure](https://image.chemsrc.com/caspic/187/65921-41-7.png)

![S-1,1-dioxide-tetrahydro-3H-Pyrrolo[1,2-c][1,2,3]oxathiazole Structure](https://image.chemsrc.com/caspic/493/132635-95-1.png)
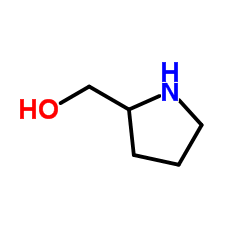
![1-[(2S)-2-Pyrrolidinylmethyl]pyrrolidine Structure](https://image.chemsrc.com/caspic/419/51207-66-0.png)