|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

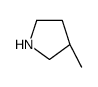
![(2R,3aS,4aR,6R,8aS)-2,6,9,9-tetramethyldecahydro-1H-benzo[e]pyrrolo[2,1-b][1,3]oxazin-1-one Structure](https://image.chemsrc.com/caspic/390/186597-26-2.png)
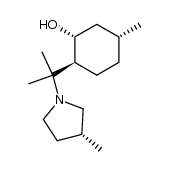

![(2S,3aS,4aR,6R,8aS)-2,6,9,9-tetramethyloctahydro-6H-benzo[e]pyrrolo[2,1-b][1,3]oxazin-1(2H)-one Structure](https://image.chemsrc.com/caspic/352/186597-27-3.png)