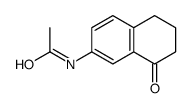
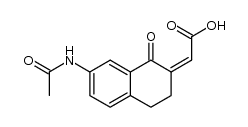
|
~72% |
|
~% |
|
~70% |
|
~% |
|
~% |
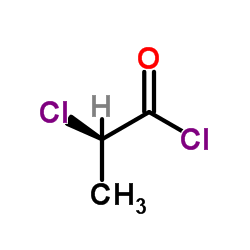

![2-chloro-N-(3-oxo-4,4a,5,6-tetrahydro-2H-benzo[h]cinnolin-8-yl)propanamide Structure](https://image.chemsrc.com/caspic/156/121866-04-4.png)
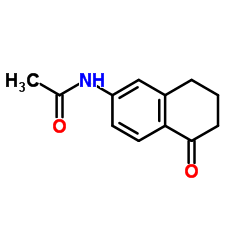
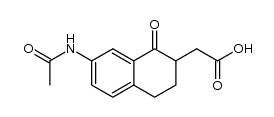
![N-(3-oxo-4,4a,5,6-tetrahydro-2H-benzo[h]cinnolin-9-yl)acetamide Structure](https://image.chemsrc.com/caspic/087/110138-93-7.png)