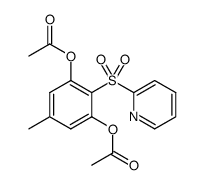
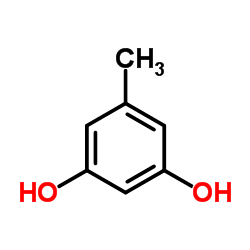
|
~91% |
|
~98% |
|
~82% |
|
~84% |
|
~34% |
|
~70% |
|
~82% |

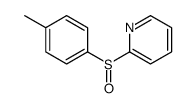
![2-[(4-methylphenyl)thiomethyl]-pyridine Structure](https://image.chemsrc.com/caspic/430/33984-18-8.png)
![2-[(4-methylphenyl)sulfonylmethyl]pyridine Structure](https://image.chemsrc.com/caspic/044/58414-96-3.png)
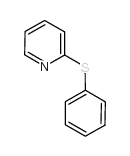
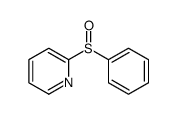

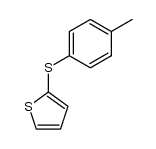
![Thiophene,2-[(4-methylphenyl)sulfonyl] Structure](https://image.chemsrc.com/caspic/167/5713-57-5.png)