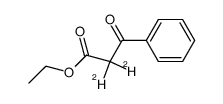
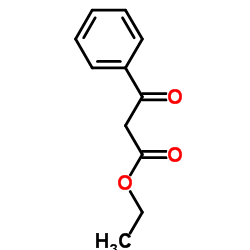
|
~87% |
|
~73% |
|
~% |
|
~% |
|
~% |
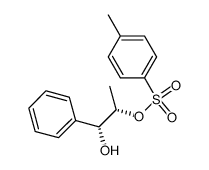

![[2-2H2]-3-chloro-1-phenylpropan-1-ol Structure](https://image.chemsrc.com/caspic/083/135561-71-6.png)
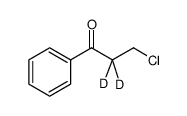
![[2-2H2]-1-phenylpropane-1,3-diol Structure](https://image.chemsrc.com/caspic/176/73738-41-7.png)