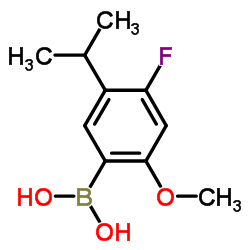
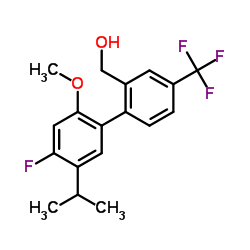
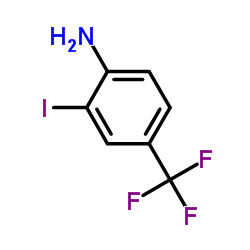
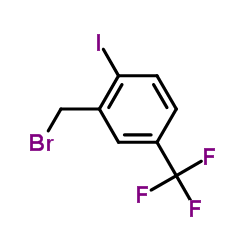
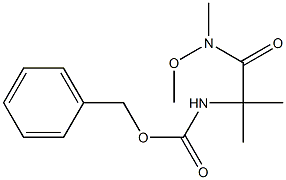
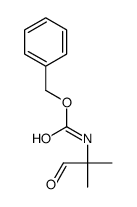
|
~% |
|
~% |
|
~% |
|
~62% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~10% |
|
~60% |
|
~% |
|
~% |
|
~% |
|
~64% |
|
~88% |
|
~% |
|
~% |
|
~% |
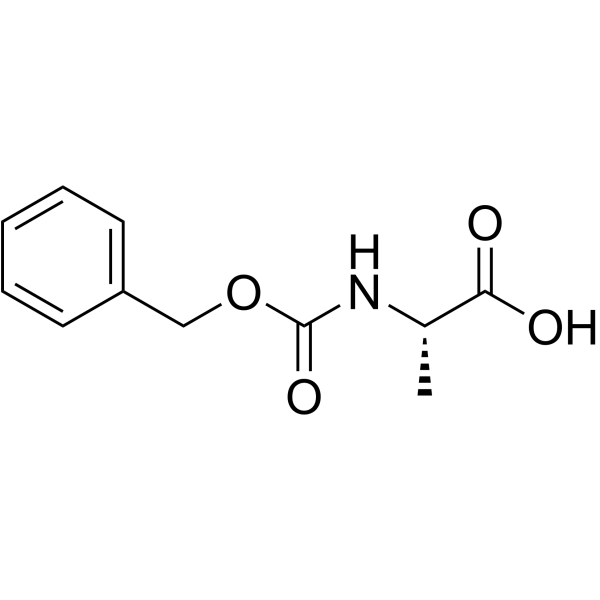
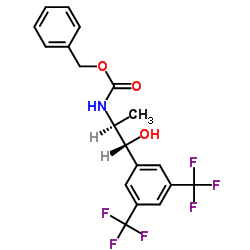
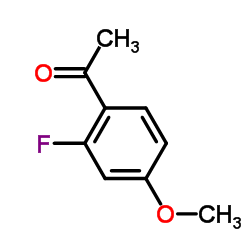



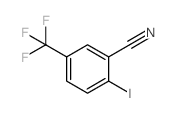
![[2-Iodo-5-(trifluoromethyl)phenyl]methanol Structure](https://image.chemsrc.com/caspic/405/702641-05-2.png)