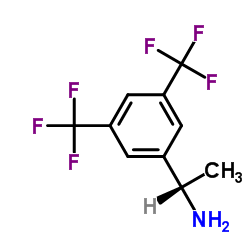
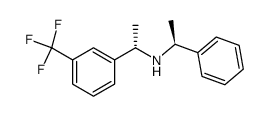
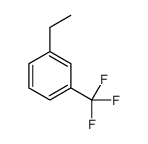
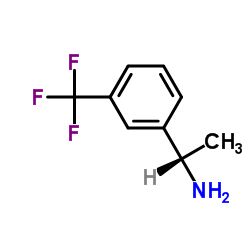
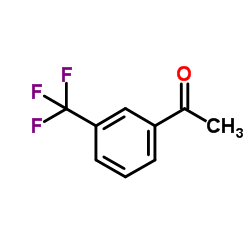
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~98% |
|
~% |
|
~% |
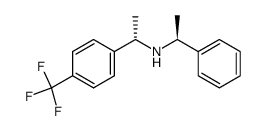
![(S)-1-[4-(Trifluoromethyl)phenyl]ethylamine Structure](https://image.chemsrc.com/caspic/228/84499-73-0.png)
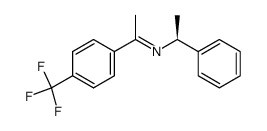
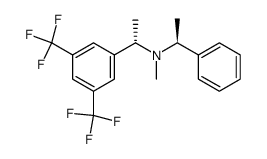
![(S)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine Structure](https://image.chemsrc.com/caspic/012/511256-36-3.png)
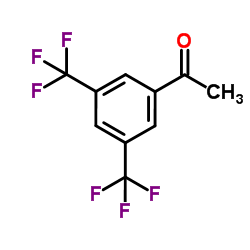
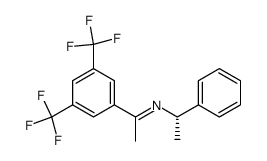
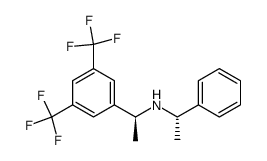
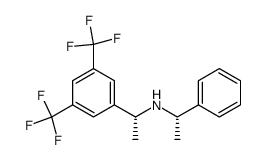
![(R)-1[3,5-Bis-(trifluoromethyl)phenyl]ethylamine Structure](https://image.chemsrc.com/caspic/279/127733-47-5.png)