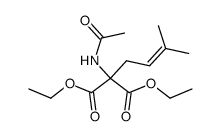
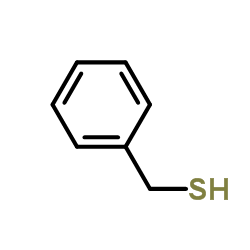
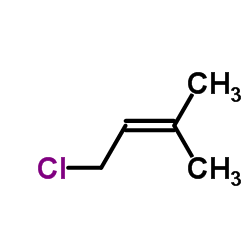
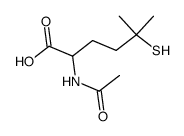
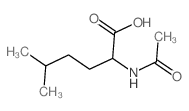
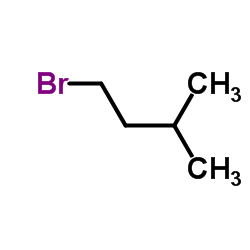
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
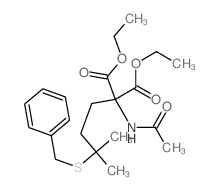
![Norleucine, N-acetyl-5-methyl-5-[(phenylmethyl)thio] Structure](https://image.chemsrc.com/caspic/159/67688-64-6.png)