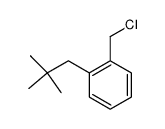
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![3-tert-Butyl-3-hydroxy-1(3H)-benzo[c]furanon Structure](https://image.chemsrc.com/caspic/007/54537-68-7.png)
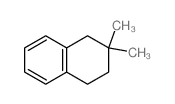
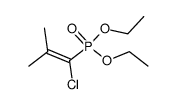

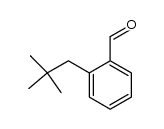
![7-tert-butylbicyclo[4.2.0]octa-1,3,5-triene Structure](https://image.chemsrc.com/caspic/077/25402-81-7.png)