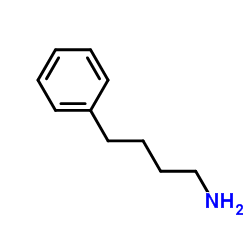
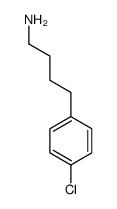
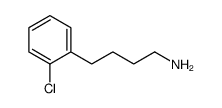
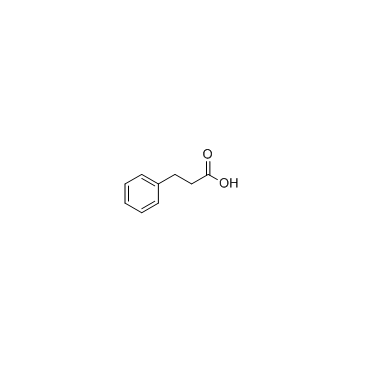
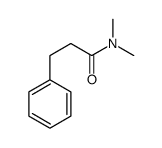
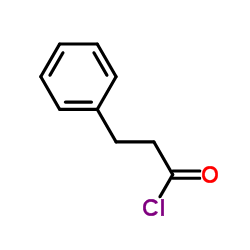
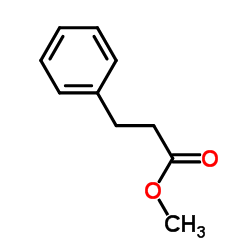
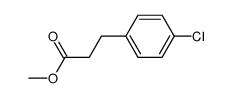
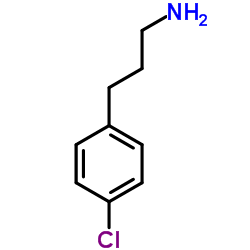
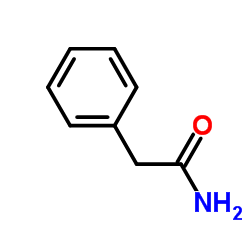
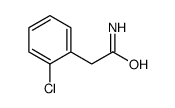
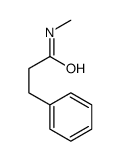
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~41% |
|
~% |
|
~% |
|
~% |
|
~% |
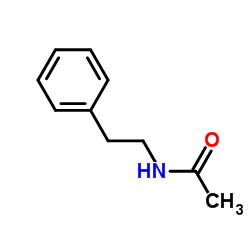
![N-[2-(4-chlorophenyl)ethyl]acetamide Structure](https://image.chemsrc.com/caspic/483/88422-94-0.png)
![N-[2-(2-chlorophenyl)ethyl]acetamide Structure](https://image.chemsrc.com/caspic/069/105871-20-3.png)