|
~% |
|
~85% |
|
~93% |
|
~86% |
|
~85% |
|
~83% |
|
~90% |
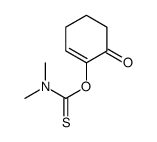
![3,5,5-trimethyl-2-[(dimethylthiocarbamoyl)oxy]-2-cyclohexen-1-one Structure](https://image.chemsrc.com/caspic/442/112621-58-6.png)
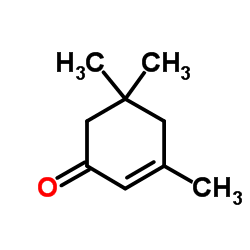
![3-methyl-2-[(dimethylthiocarbamoyl)oxy]-2-cyclopenten-1-one Structure](https://image.chemsrc.com/caspic/036/110874-84-5.png)
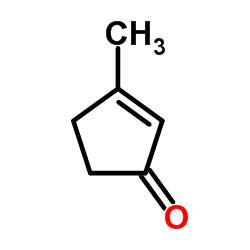
![3-tert-butyl-2-[(dimethylthiocarbamoyl)oxy]-2-cyclopenten-1-one Structure](https://image.chemsrc.com/caspic/329/110874-94-7.png)
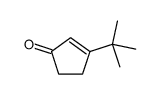
![3-methyl-2-[(dimethylthiocarbamoyl)oxy]-2-cyclohexen-1-one Structure](https://image.chemsrc.com/caspic/480/112621-57-5.png)
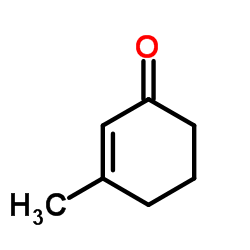
![6-isopropyl-3-methyl-2-[(dimethylthiocarbamoyl)oxy]-2-cyclohexen-1-one Structure](https://image.chemsrc.com/caspic/001/112621-61-1.png)
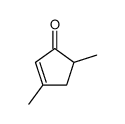
![3,5-dimethyl-2-[(dimethylthiocarbamoyl)oxy]-2-cyclopenten-1-one Structure](https://image.chemsrc.com/caspic/367/110874-93-6.png)