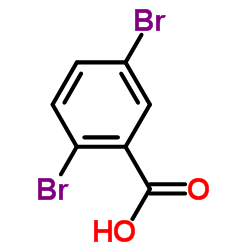
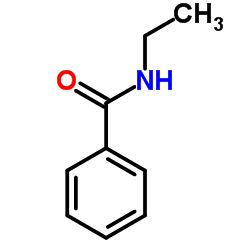
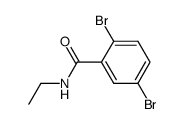
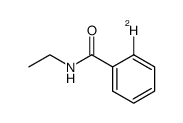
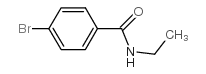
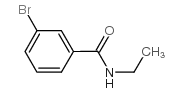
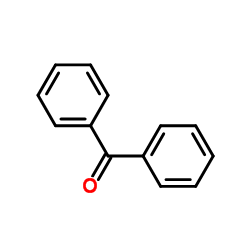
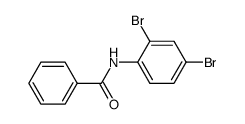
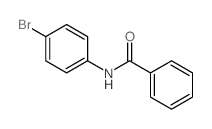
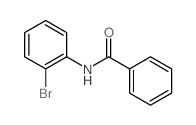
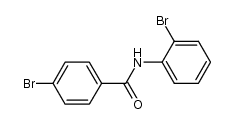
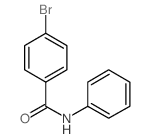
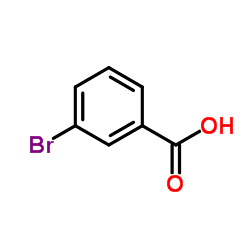
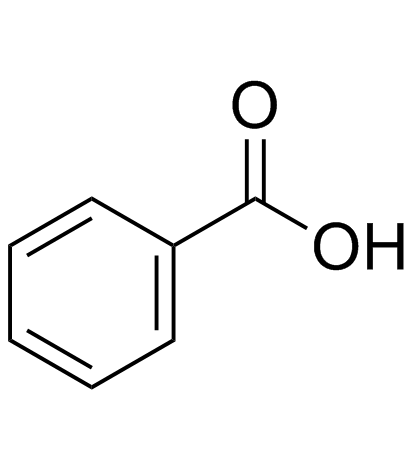
|
~% |
|
~% |
|
~% |
|
~% |
|
~36% |
|
~39% |
|
~47% |
|
~41% |
|
~% |
|
~% |
|
~62% |
|
~% |
|
~% |
|
~4% |
|
~40% |
|
~13% |
|
~5% |
|
~21% |