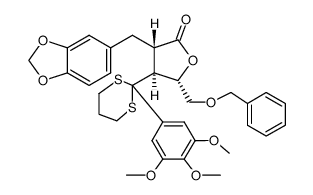
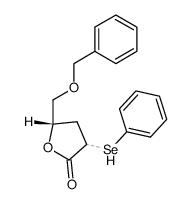
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |
![3-(1,3-benzodioxol-5-ylmethyl)-5-(hydroxymethyl)-4-[(3,4,5-trimethoxyphenyl)methyl]oxolan-2-one Structure](https://image.chemsrc.com/caspic/219/72627-52-2.png)
![(2S,3S,4S)-4-(1,3-benzodioxol-5-ylmethyl)-3-[(3,4,5-trimethoxyphenyl)methyl]pentane-1,2,5-triol Structure](https://image.chemsrc.com/caspic/212/76236-36-7.png)
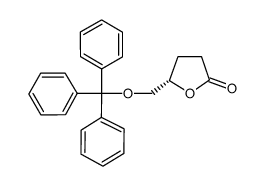
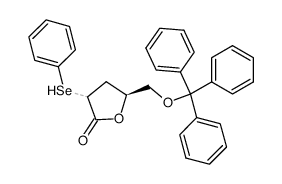
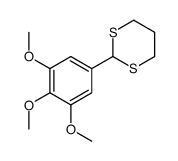

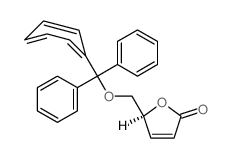
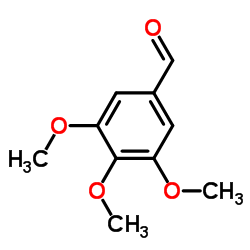
![(4S,5S)-5-(hydroxymethyl)-4-[(3,4,5-trimethoxyphenyl)methyl]oxolan-2-one Structure](https://image.chemsrc.com/caspic/250/76236-35-6.png)
![D-erythro-Pentonicacid,2,3-dideoxy-3-[2-(3,4,5-trimethoxyphenyl)-1,3-dithian-2-yl]-5-O-(triphenylmethyl)-,g-lactone Structure](https://image.chemsrc.com/caspic/326/76236-33-4.png)
![2(3H)-Furanone, dihydro-5-[(phenylmethoxy)methyl]-, (5S) Structure](https://www.chemsrc.com/extcaspic/328/32780-08-8.png)