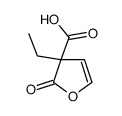
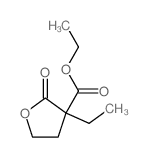
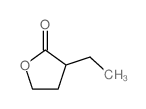
|
~71% |
|
~22% |
|
~88% |
|
~99% |
|
~39% |


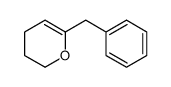
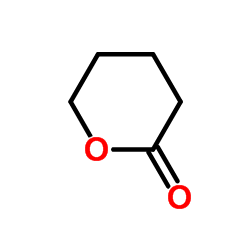
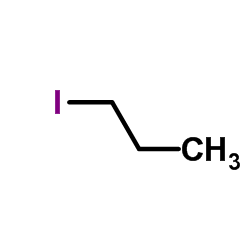

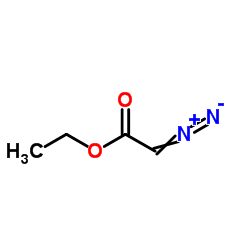
![ethyl 2-oxabicyclo[4.1.0]heptane-7-carboxylate Structure](https://image.chemsrc.com/caspic/086/72229-08-4.png)