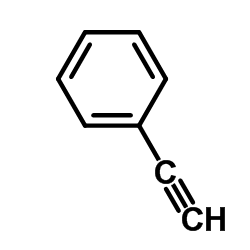
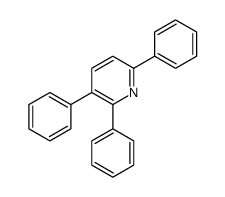
Facile selective synthesis of new furo [3, 4-d]-1, 3-thiazol...
[Aly, Ashraf A.; Hassan, Alaa A.; Al-Qalawi, Husam R.M.; Ishak, Esam A. Journal of Sulfur Chemistry, 2012 , vol. 33, # 4 p. 419 - 426]
|
Facile selective synthesis of new furo [3, 4-d]-1, 3-thiazol...
[Aly, Ashraf A.; Hassan, Alaa A.; Al-Qalawi, Husam R.M.; Ishak, Esam A. Journal of Sulfur Chemistry, 2012 , vol. 33, # 4 p. 419 - 426]
|
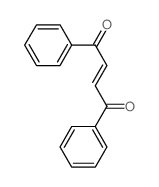
Facile selective synthesis of new furo [3, 4-d]-1, 3-thiazol...
[Aly, Ashraf A.; Hassan, Alaa A.; Al-Qalawi, Husam R.M.; Ishak, Esam A. Journal of Sulfur Chemistry, 2012 , vol. 33, # 4 p. 419 - 426]
|
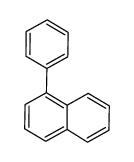
Facile selective synthesis of new furo [3, 4-d]-1, 3-thiazol...
[Aly, Ashraf A.; Hassan, Alaa A.; Al-Qalawi, Husam R.M.; Ishak, Esam A. Journal of Sulfur Chemistry, 2012 , vol. 33, # 4 p. 419 - 426]
|
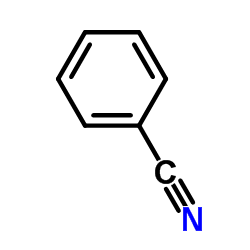
Diazo Decomposition in the Presence of Tributyltin Hydride. ...
[Tan, Zhongping; Qu, Zhaohui; Chen, Bei; Wang, Jianbo Tetrahedron, 2000 , vol. 56, # 38 p. 7457 - 7461]
|