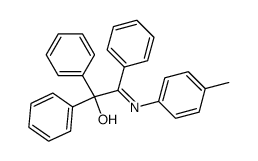
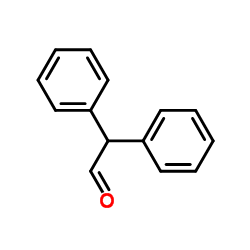
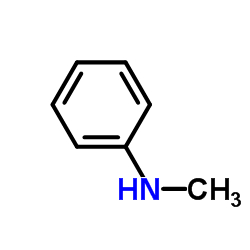
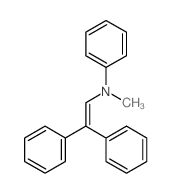
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
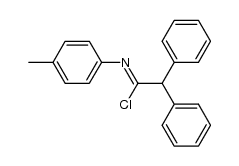

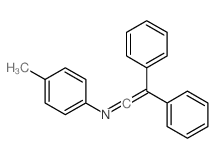

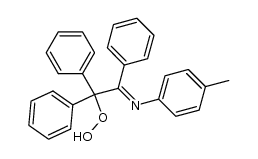
![2-[(4-methylphenyl)amino]-1,1,2-triphenyl-ethanol Structure](https://image.chemsrc.com/caspic/062/5469-99-8.png)