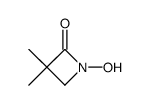
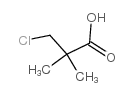
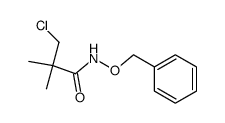
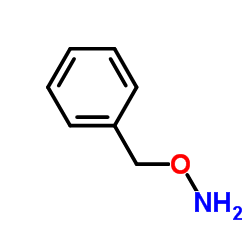
|
~98% |
|
~79% |
|
~99% |
|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~60% |

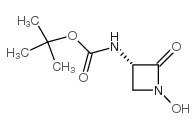
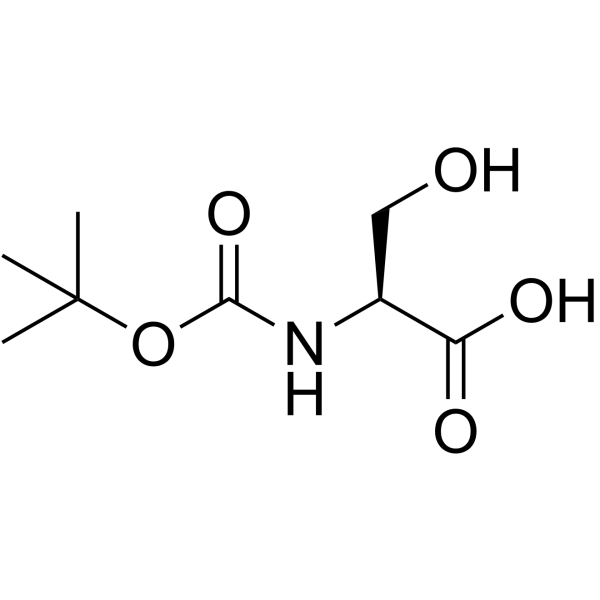
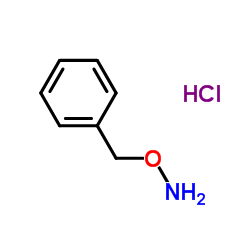
![Carbamic acid,N-[(1S)-1-(hydroxymethyl)-2-oxo-2-[(phenylmethoxy)amino]ethyl]-,1,1-dimethylethyl ester Structure](https://image.chemsrc.com/caspic/445/26048-92-0.png)