|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
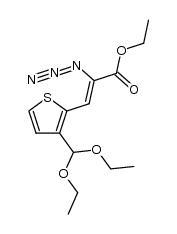
![5-oxo-9-thia-4-azabicyclo[4.3.0]nona-2,7,10-triene-3-carboxylic acid Structure](https://image.chemsrc.com/caspic/024/60249-02-7.png)
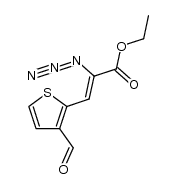

![4-ethoxy-thieno[3,2-c]pyridine-6-carboxylic acid ethyl ester Structure](https://image.chemsrc.com/caspic/100/60249-00-5.png)
![5-ethoxy-9-thia-4-azabicyclo[4.3.0]nona-1,3,5,7-tetraene-3-carboxylic acid Structure](https://image.chemsrc.com/caspic/448/60249-04-9.png)
![thieno[3,2-c]pyridine-6-carboxylic acid Structure](https://image.chemsrc.com/caspic/248/60249-09-4.png)
![Thieno[3,2-c]pyridine Structure](https://image.chemsrc.com/caspic/402/272-14-0.png)

![thieno[3,2-c]pyridin-4-ol Structure](https://image.chemsrc.com/caspic/174/27685-92-3.png)
![7-oxo-6,7-dihydro-thieno[2,3-c]pyridine-5-carboxylic acid Structure](https://image.chemsrc.com/caspic/062/60249-01-6.png)
![THIENO[2,3-C]PYRIDIN-7(6H)-ONE Structure](https://image.chemsrc.com/caspic/453/28981-13-7.png)
![Thieno[2,3-c]pyridine-5-carboxylic acid, 7-ethoxy Structure](https://image.chemsrc.com/caspic/486/60249-03-8.png)
![Thieno[2,3-c]pyridine-5-carboxylic acid Structure](https://image.chemsrc.com/caspic/286/60249-08-3.png)
![Thieno[2,3-c]pyridine Structure](https://image.chemsrc.com/caspic/488/272-12-8.png)
![thieno[2,3-c]pyridine-5-carboxylic acid ethyl ester Structure](https://image.chemsrc.com/caspic/362/60249-06-1.png)