|
~80% |
|
~84% |
|
~95% |
|
~91% |
|
~89% |
|
~82% |
|
~95% |
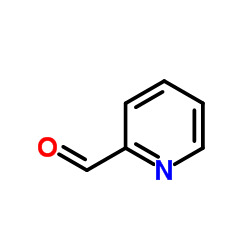
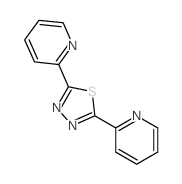
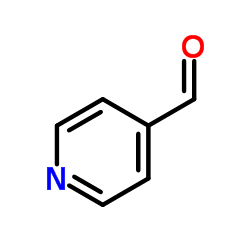


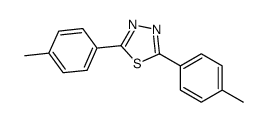
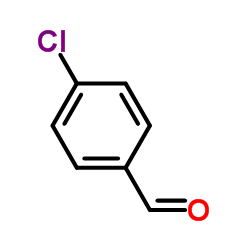


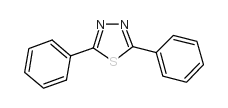
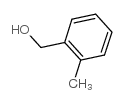
![6-[5-(6-oxocyclohexa-2,4-dien-1-ylidene)-1,3,4-thiadiazolidin-2-ylidene]cyclohexa-2,4-dien-1-one Structure](https://image.chemsrc.com/caspic/244/88203-22-9.png)
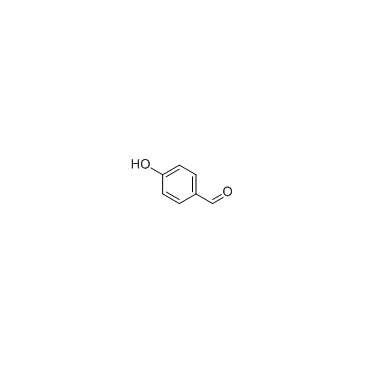
![4-[5-(4-oxocyclohexa-2,5-dien-1-ylidene)-1,3,4-thiadiazolidin-2-ylidene]cyclohexa-2,5-dien-1-one Structure](https://image.chemsrc.com/caspic/196/88203-23-0.png)