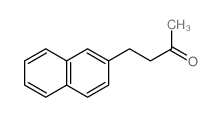
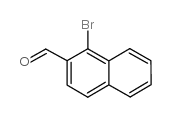
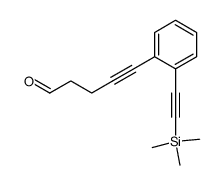
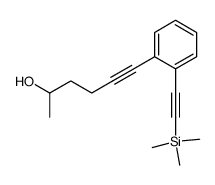
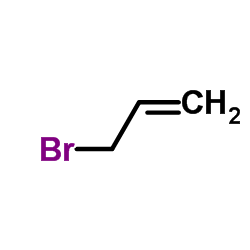
|
~49% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~52% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~5% |
|
~40% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

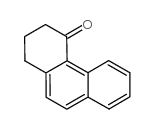
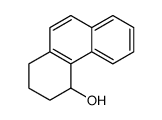

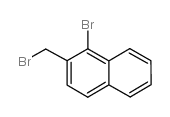

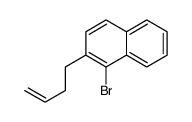
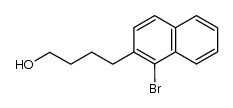
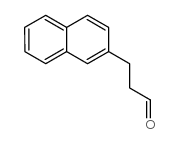
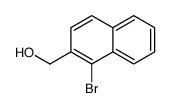

![2,3-DIHYDRO-1H-BENZ[E]INDEN-1-ONE Structure](https://image.chemsrc.com/caspic/426/6342-87-6.png)