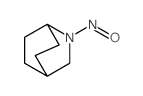
|
~94% |
|
~% |
|
~% |
|
~% |
|
~98% |
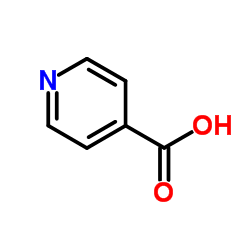

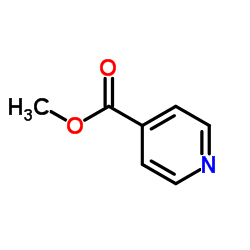
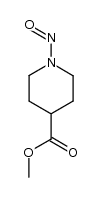
![1,2-diazabicyclo[2.2.2]octan-3-one Structure](https://image.chemsrc.com/caspic/468/1632-26-4.png)
![2-AZABICYCLO[2.2.2]OCTANE Structure](https://image.chemsrc.com/caspic/213/280-38-6.png)