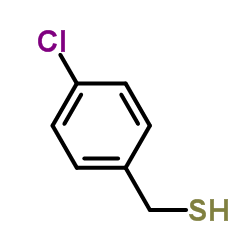
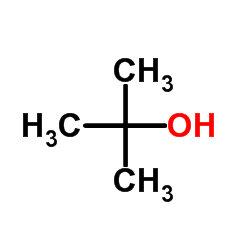
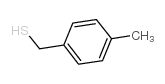
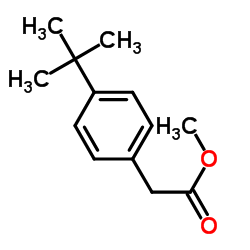
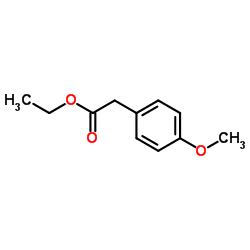
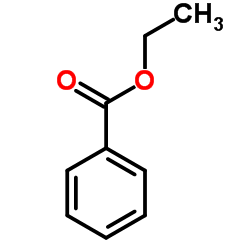
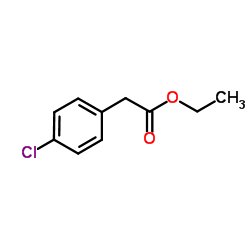
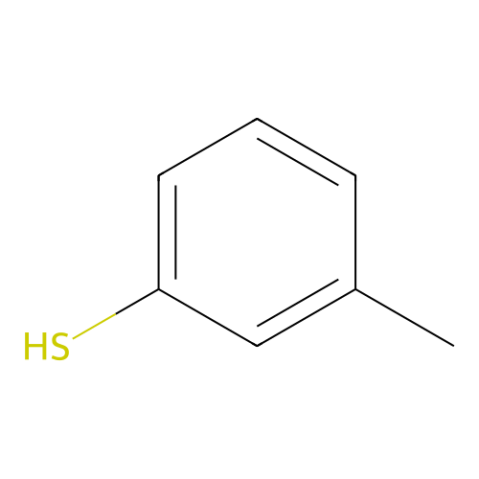
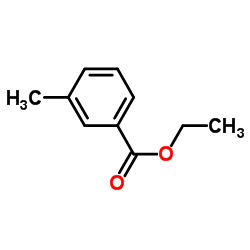
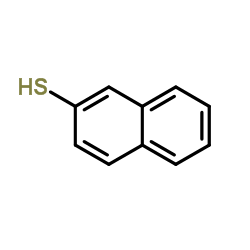
|
~58% |
|
~27% |
|
~72% |
|
~68% |
|
~31% |
|
~64% |
|
~77% |
|
~50% |