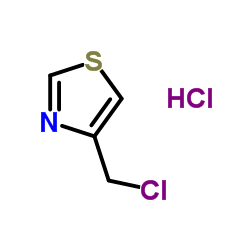
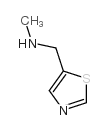
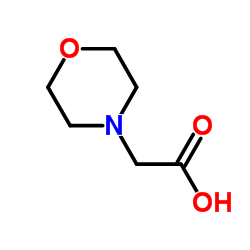
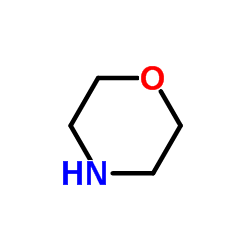
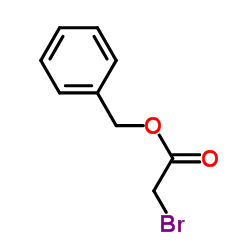
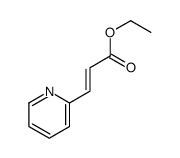
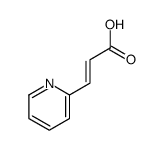
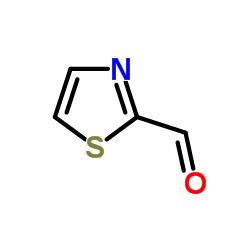
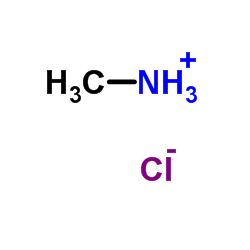
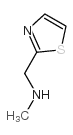
|
~97% |
|
~95% |
|
~90% |
|
~91% |
|
~18% |