|
~77% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
![[2S-[2α(E),3β,4β,5α(2E,4S*,5R*)]]-9-[[3-methyl-1-oxo-4-[tetrahydro-3,4-dihydroxy-5-(5-hydroxy-4-methyl-2-hexenyl)-2H-pyran-2-yl]-2-butenyl]oxy]nonanoic acid methyl ester Structure](https://image.chemsrc.com/caspic/042/72042-22-9.png)
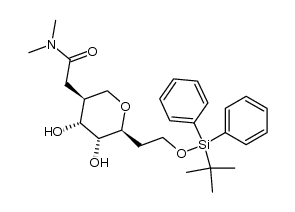

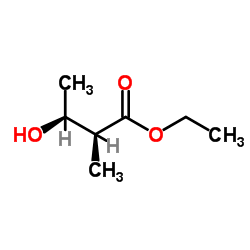

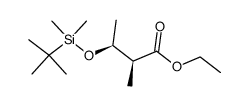

![2,7-dioxabicyclo[4.3.0]non-4-en-8-one Structure](https://image.chemsrc.com/caspic/268/115183-65-8.png)
![(3aS,7R,7aR)-7-iodohexahydro-2H-furo[3,2-b]pyran-2-one Structure](https://image.chemsrc.com/caspic/306/115183-64-7.png)

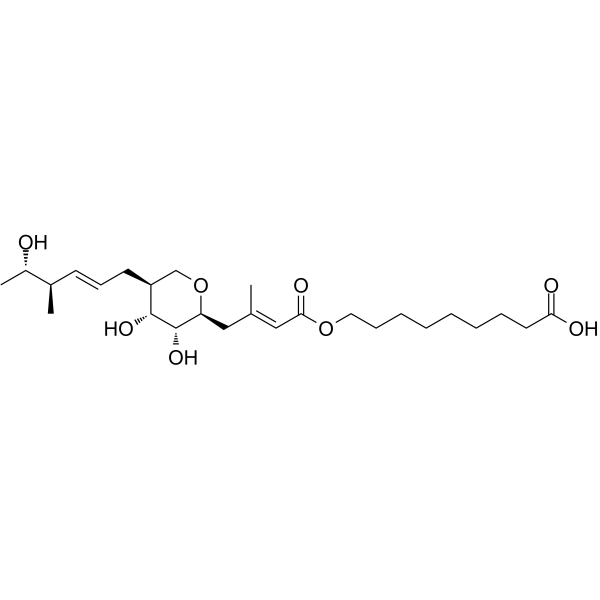
![(2S-cis)-5,6-dihydro-N,N-dimethyl-2-[2-[[(1,1-dimethylethyl)diphenylsilyl]oxy]ethyl]-2H-pyran-5-acetamide Structure](https://image.chemsrc.com/caspic/375/115140-92-6.png)
![[S-(R*,R*)]-5,6-dihydro-N-(1-phenylethyl)-2H-pyran-2-acetamide Structure](https://image.chemsrc.com/caspic/476/115118-79-1.png)
![(5S-cis)-5,6-dihydro-6-[2-[[(1,1-dimethylethyl)diphenylsilyl]oxy]ethyl]-2H-pyran-5-ol Structure](https://image.chemsrc.com/caspic/378/107148-23-2.png)
![(2S,3S)-3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methyl-1-(phenylsulfonyl)butane Structure](https://image.chemsrc.com/caspic/431/115118-85-9.png)
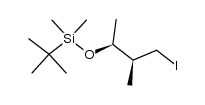
![(S)-3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methyl-1-butanol 4-methylbenzenesulfonate Structure](https://image.chemsrc.com/caspic/007/115118-83-7.png)
![(2R,3R,E)-2-((tert-butyldimethylsilyl)oxy)-6-((3aS,4S,7S,7aR)-4-(2-((tert-butyldiphenylsilyl)oxy)ethyl)-2,2-dimethyltetrahydro-3aH-[1,3]dioxolo[4,5-c]pyran-7-yl)-3-methylhex-4-en-1-ol Structure](https://image.chemsrc.com/caspic/307/115118-88-2.png)
![tert-butyl(2-((3aS,4S,7S,7aR)-7-((4R,5R,E)-5-((tert-butyldimethylsilyl)oxy)-6-iodo-4-methylhex-2-en-1-yl)-2,2-dimethyltetrahydro-3aH-[1,3]dioxolo[4,5-c]pyran-4-yl)ethoxy)diphenylsilane Structure](https://image.chemsrc.com/caspic/269/115118-89-3.png)


![benzyl 2-[tert-butyl(dimethyl)silyl]oxyacetate Structure](https://image.chemsrc.com/caspic/383/115118-86-0.png)

