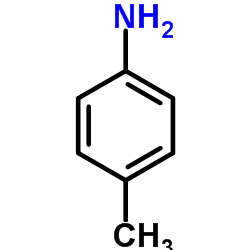
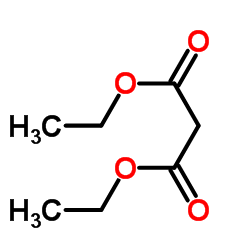
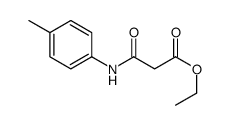
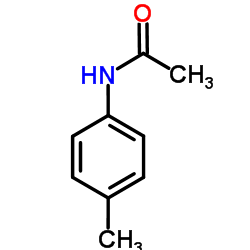
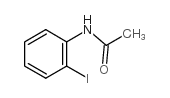
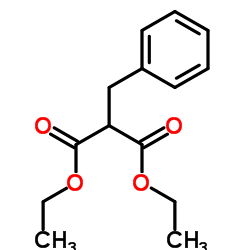
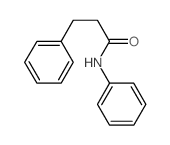
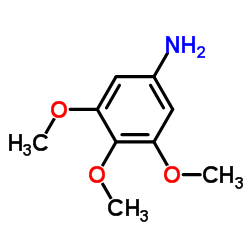
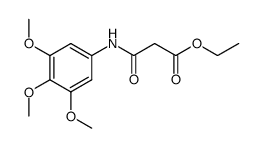
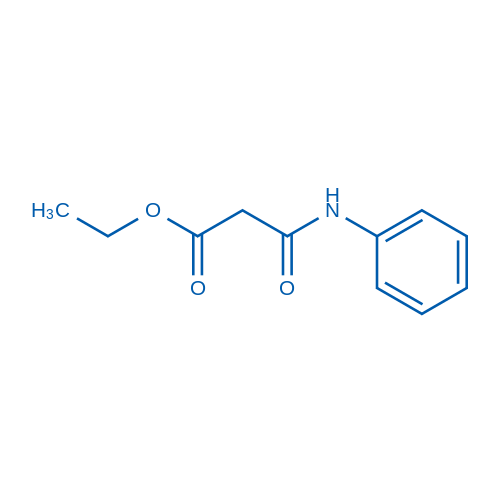
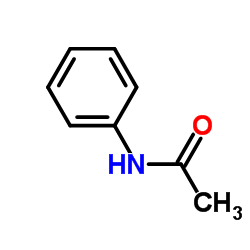
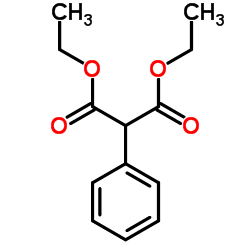
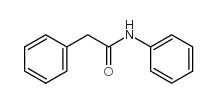
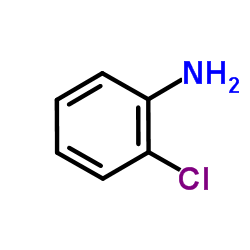
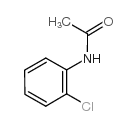
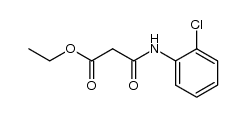
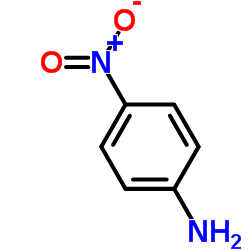
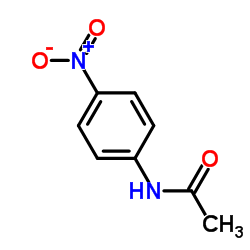
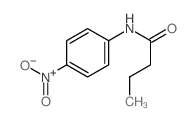
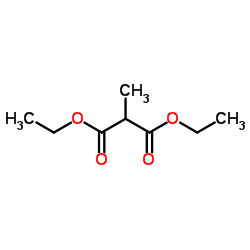
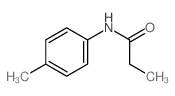
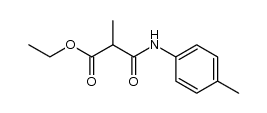
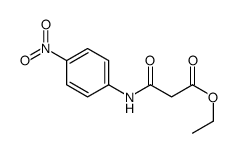
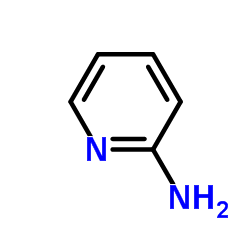
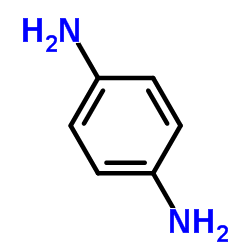
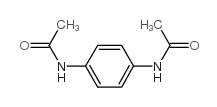
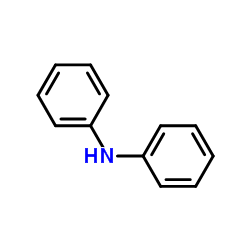
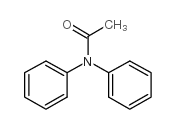
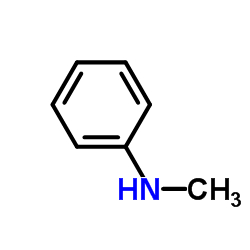
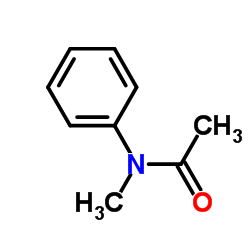
|
~% |
|
~65% |
|
~85% |
|
~74% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~98% |
|
~40% |
|
~10% |
|
~61% |
|
~% |
|
~67% |
|
~% |
|
~72% |
|
~% |
|
~87% |
|
~68% |
|
~76% |
|
~93% |
|
~% |
|
~92% |