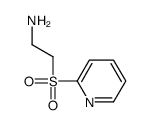
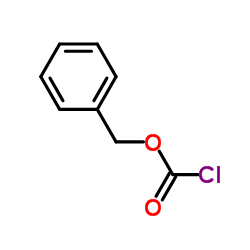
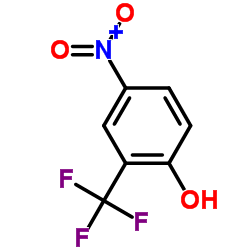
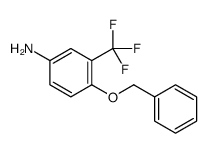
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![[2-(Methylsulfonyl)ethyl]carbamic Acid Benzyl Ester Structure](https://image.chemsrc.com/caspic/013/146698-94-4.png)
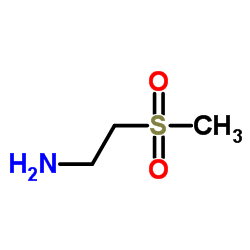
![[2-(Pyridin-2-ylthio)ethyl]amine dihydrochloride Structure](https://image.chemsrc.com/caspic/237/42416-20-6.png)