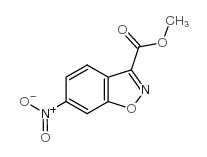
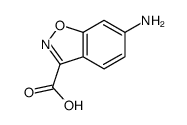
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
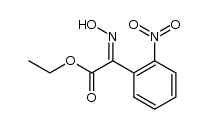

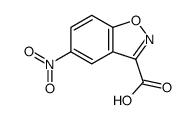
![Ethyl benzo[d]isoxazole-3-carboxylate Structure](https://image.chemsrc.com/caspic/255/57764-49-5.png)