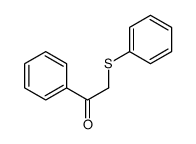
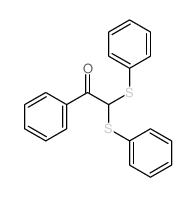
|
~% |
|
~38%
Detail
|
|
~% |
|
~% |
|
~% |
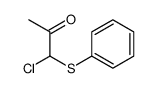
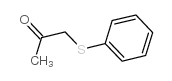

![3-methyl-2-(phenylthio)benzo[b]thiophene Structure](https://image.chemsrc.com/caspic/431/77128-60-0.png)