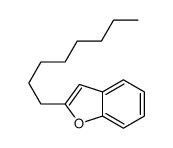
|
~38% |
|
~% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~32% |
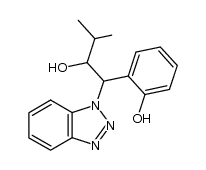

![1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-methyl-1-(2-((trimethylsilyl)oxy)phenyl)butan-2-ol Structure](https://image.chemsrc.com/caspic/496/1028281-48-2.png)



![4-phenyl-3,4-dihydro-1H-benzo[f]isoquinolin-2-one Structure](https://image.chemsrc.com/caspic/138/78634-33-0.png)