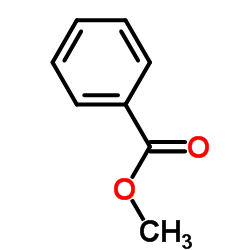
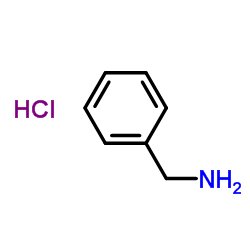
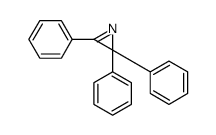
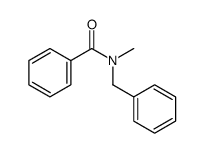
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~% |
|
~96% |

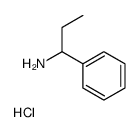
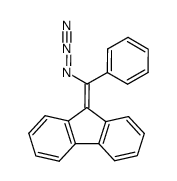
![3-phenylspiro[azirine-2,9'-fluorene] Structure](https://image.chemsrc.com/caspic/267/79893-70-2.png)

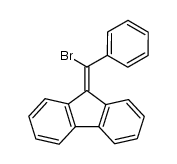
![9-Bromo-9-[bromo(phenyl)methyl]-9H-fluorene Structure](https://image.chemsrc.com/caspic/110/79898-41-2.png)