|
~23% |
|
~22% |
|
~63% |
|
~37% |
|
~37% |
|
~9% |
|
~45% |
|
~50% |
|
~6% |
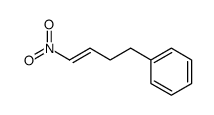
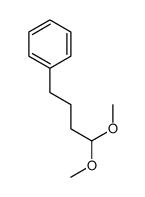
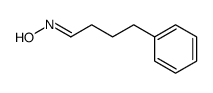

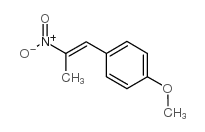

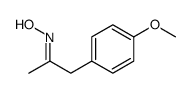
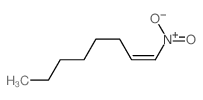
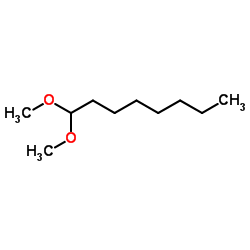
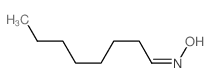
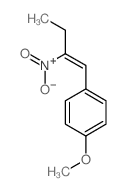
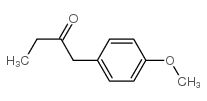
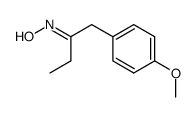
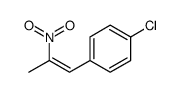
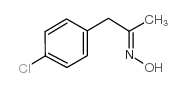
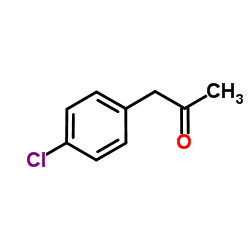
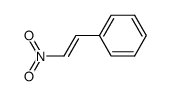
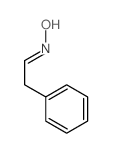
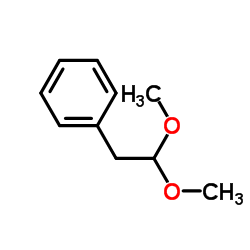
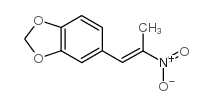
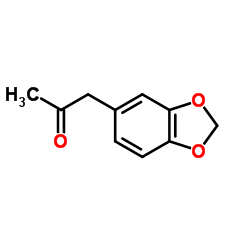
![benzo[1,3]dioxol-5-yl-acetone oxime Structure](https://image.chemsrc.com/caspic/171/136056-99-0.png)