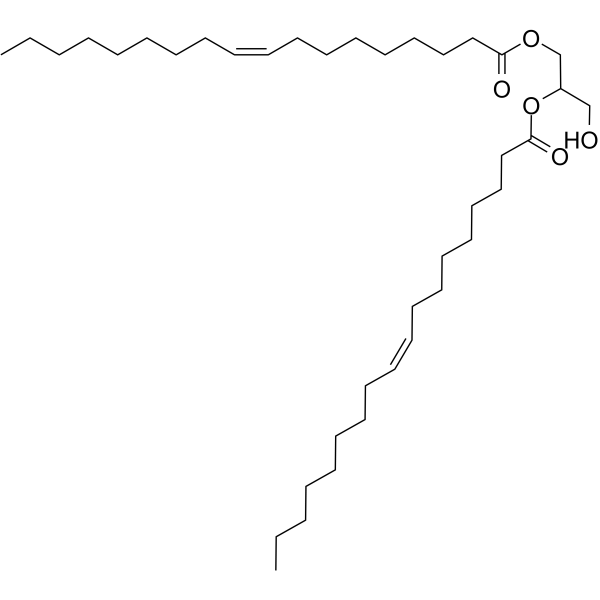
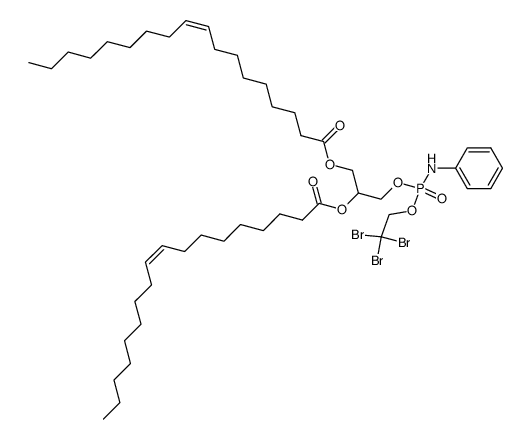
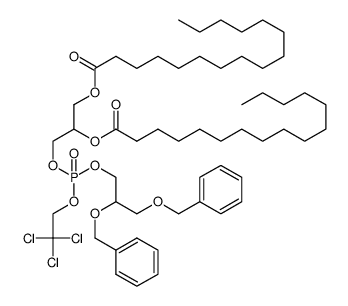
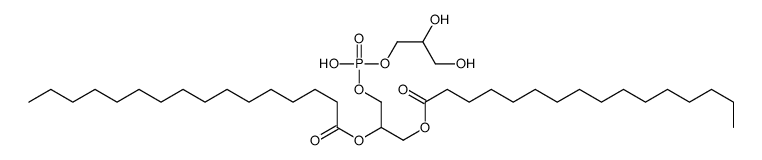
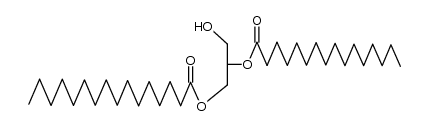
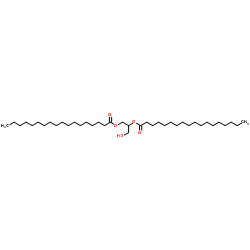
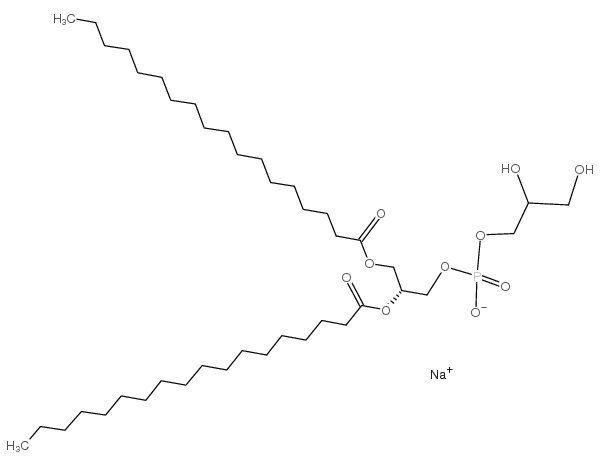
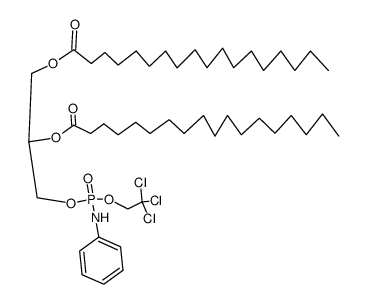
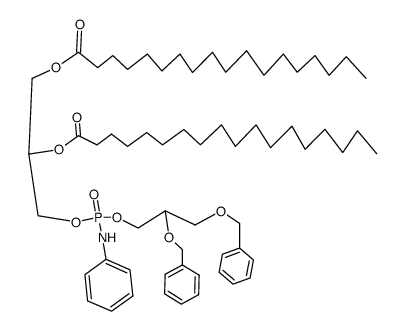
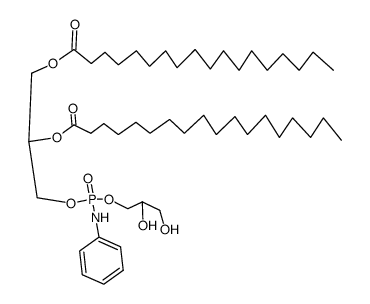
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
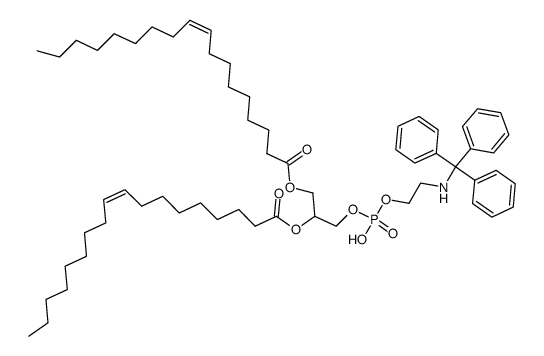
![[1-(2-aminoethoxy-hydroxyphosphoryl)oxy-3-[(Z)-octadec-9-enoyl]oxypropan-2-yl] (Z)-octadec-9-enoate Structure](https://image.chemsrc.com/caspic/162/2462-63-7.png)