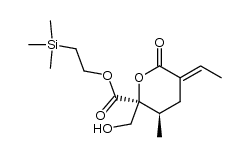
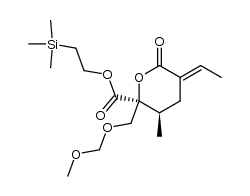
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~% |
![(4R)-4-[(4S)-2,2-Dimethyl-4-[[2-(trimethylsilyl)ethoxy]carbonyl]-1,2-dioxolanyl]pentanoic Acid Structure](https://image.chemsrc.com/caspic/009/122445-41-4.png)

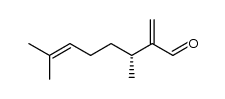
![(4R)-2,2-Dimethyl-4-[1(1R)-1,5-dimethyl-4-hexenyl]-4-(hydroxymethyl)-1,3-dioxolane Structure](https://image.chemsrc.com/caspic/450/122445-38-9.png)
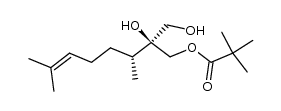
![(4R,5S)-5-[[2-(Trimethylsilyl)ethoxy]carbonyl]-5-(hydroxymethyl)-4-methyl-5-pentanolide Structure](https://image.chemsrc.com/caspic/433/122445-43-6.png)
![(4S)-2,2-Dimethyl-4-[(1R)-1,5-dimethyl-4-hexenyl]-4-[[2-(trimethylsilyl)ethoxy]carbonyl]-1,3-dioxolane Structure](https://image.chemsrc.com/caspic/047/122445-40-3.png)
![(4S)-2,2-Dimethyl-4-[1(1R)-1,5-dimethyl-4-hexenyl]-4-[(pivalyloxy)methyl]-1,3-dioxolane Structure](https://image.chemsrc.com/caspic/488/122445-37-8.png)