|
~98% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~67% |
|
~95% |
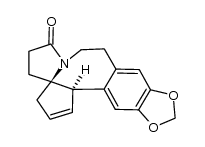
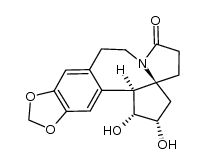
![1-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-1-azaspiro[4.4]nonane-2,6-dione Structure](https://image.chemsrc.com/caspic/491/114942-69-7.png)
![N-[2-[3,4-(methylenedioxy)phenyl]ethyl]-6α-hydroxy-2-oxo-1β-azaspiro[4.4]non-7-ene Structure](https://image.chemsrc.com/caspic/360/114942-77-7.png)

![(5S,6S)-1-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-2-oxo-1-azaspiro[4.4]non-7-en-6-yl acetate Structure](https://image.chemsrc.com/caspic/322/114942-78-8.png)
![N-[2-[3,4-(methylenedioxy)phenyl]ethyl]-2,6-dioxo-1-azaspiro[4.4]non-7-ene Structure](https://image.chemsrc.com/caspic/474/114942-74-4.png)
![1-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-7-(phenylselanyl)-1-azaspiro[4.4]nonane-2,6-dione Structure](https://image.chemsrc.com/caspic/398/114942-76-6.png)