|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~91% |
|
~96% |
|
~% |
![methyl N-(methoxymethyl)pyrido[3,4-b]indole-3-carboxylate Structure](https://image.chemsrc.com/caspic/023/1040742-77-5.png)
![methyl 1-chloro-N-(methoxymethyl)pyrido[3,4-b]indole-3-carboxylate Structure](https://image.chemsrc.com/caspic/044/1040742-81-1.png)
![methyl 3-[2-formyl-N-(methoxymethyl)indol-3-yl]acrylate Structure](https://image.chemsrc.com/caspic/447/1040742-79-7.png)
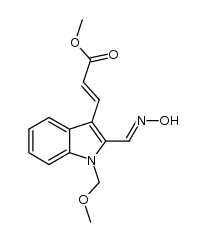
![3-(methoxycarbonyl)-9-(methoxymethyl)-9H-pyrido[3,4-b]indole 2-oxide Structure](https://image.chemsrc.com/caspic/082/1040742-80-0.png)