|
~93% |
|
~97% |
|
~0% |
|
~48% |
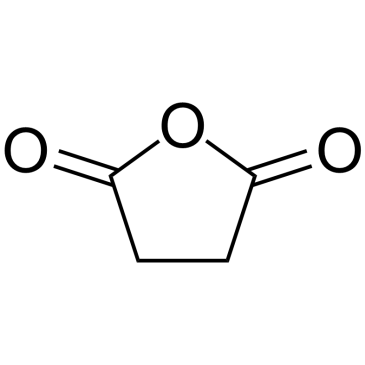
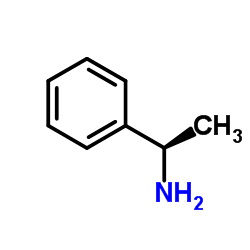



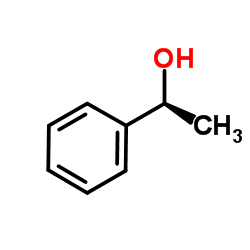
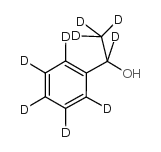

![N,N'-bis[(1S)-1-phenylethyl]propane-1,3-diamine Structure](https://image.chemsrc.com/caspic/044/157488-66-9.png)